Document Management System in Quality Assurance Department Standard Operating Procedure (SOP) Objective: To lay down a procedure for Management of Documents in Quality Assurance Department in Pharma Industry Scope: Applicable for Document Management system in Quality Assurance department. Responsibility: Quality Assurance Department Accountability: Head- QA shall be accountable for compliance …
Read More »SOP for operation of rapid mixer granulator (RMG) with cone mill in Pharma Industry
SOP for operation of rapid mixer granulator (RMG) with cone mill in Pharma Industry Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of rapid mixer granulator with cone mill in Pharma Industry Scope This SOP is applicable for operation of rapid mixer granulator with cone mill …
Read More »Cleaning of Rapid Mixer Granulator with Cone Mill in Pharma Industry
Cleaning of Rapid Mixer Granulator with Cone Mill in Pharma Industry Standard Operating Procedure (SOP) Objective: To lay down a procedure for cleaning of Rapid Mixer Granulator with cone mill in Pharma Industry. Scope: This SOP is applicable for the cleaning of Rapid Mixer Granulator with cone mill located in …
Read More »SOP on cleaning of Paste Kettle in Pharma Industry
SOP on cleaning of Paste Kettle in Pharma Industry Standard Operating Procedure (SOP) Objective To lay down a procedure for cleaning of Paste Kettle in Pharma Industry. Scope This SOP is applicable for the cleaning of Paste Kettle in the formulation plant of Pharma Industry. Responsibility Production Operator/ Technician – …
Read More »SOP for operation of Metal Detector in Pharma Industry
SOP for operation of Metal Detector in Pharma Industry Standard Operating Procedure (SOP) Objective: To lay down a procedure for operation of metal detector in Pharma Industry. Scope: This SOP is applicable for the operation of metal detector to the formulation plant of Pharma Industry. Responsibility: Production Operator/ Technician shall be …
Read More »SOP on Operation of Paste Kettle In Pharma Industry
SOP on the operation of Paste Kettle In the Pharma Industry Objective To lay down the procedure for operation of Paste Kettle In Pharma Industry. Scope This SOP is applicable for operation of Paste Kettle In Pharma Industry. Responsibility Production Operator/ Technician – For operation of the equipment. Production Officer/ …
Read More »Contract production, analysis and other activities as per WHO Guideline in Pharma Industry
Contract production, analysis, and other activities in the Pharma Industry Principle. Contract production, analysis and any other activity covered by GMP must be correctly defined, agreed and controlled in order to avoid misunderstandings that could result in a product, or work or analysis, of unsatisfactory quality. All arrangements for contract …
Read More »Pharma Manufacturing Premises – Risk classification observations
Pharma Manufacturing Premises – Risk classification guide for drug good manufacturing practices observations Risk 1 (critical) observations in Pharma Industry There was no air filtration system to eliminate airborne contaminants likely to be generated during fabrication or packaging. There was generalized malfunctioning of the ventilation system(s), with evidence of widespread …
Read More »Process Validation: General Principles (USFDA) in Pharma Industry
Process Validation: General Principles (USFDA) in Pharma Industry This guidance incorporates principles and approaches that all manufacturers can use to validate manufacturing processes. FDA encourages the use of modern pharmaceutical development concepts, quality risk management, and quality systems at all stages of the manufacturing process lifecycle. The lifecycle concept links …
Read More »Management Review
Management Review Management Review has a systematic approach for quality review as per the Quality Management System (QMS) to monitor product quality, validate the status of products and related processes, identify improvements required in manufacturing processes and systems, and take care of quality risks throughout product life-cycle. Scope of Management …
Read More »SOP on SOP in Industry (Pharmaceuticals Industry)
Standard Operating Procedures- SOP on SOP in Industry (Pharmaceuticals Industry) SOP in Industry stands for “standard operating procedure,” a set of step-by-step instructions for completing a task, An SOP goes beyond being just a procedural document it serves as a reference guide for problem-solving, a tool for ensuring safety, and …
Read More »Line Clearance
line clearance Objective: To lay down the procedure for line clearance of manufacturing and warehouse area. This is to ensure that the start up of any production/ process is free from previous material/ contaminants. Scope: This SOP is applicable for line clearance of production (oral), production (Injection), and warehouse areas …
Read More »PREVENTIVE MAINTENANCE OF PRODUCTION EQUIPMENT
PREVENTIVE MAINTENANCE OF PRODUCTION EQUIPMENT OBJECTIVE; To define the procedure for preventive maintenance of production equipment. SCOPE: This procedure is applicable for preventive maintenance of production equipment. RESPONSIBILITY: This procedure is applicable for preventive maintenance of production equipment. RESPONSIBILITY: Mechanical Engineer ACCOUNTABILITY: Engineer Head ATTACHMENTS: Attachment I – Preventive maintenance …
Read More »Indian Pharmacopoeia Commission (IPC)
Indian Pharmacopoeia Commission (IPC) In the healthcare system, Indian Pharmacopoeia Commission (IPC) plays an important role particularly in matters related to the quality standards, safety and rational use of medicines in the country. IPC was set up on 01st January, 2009 as an autonomous Institution under the Ministry of Health …
Read More »SOP for Procedure of shutdown and startup of Facility
SOP for Procedure of shutdown and startup of Facility Objective To lay down the procedure of shutdown and start-up after shutdown of Facility. Scope This SOP is applicable for procedure of shutdown and start up after shutdown of Facility in the formulation plant. Responsibility All personnel working in respective areas. …
Read More »SCALE UP AND POSTAPPROVAL CHANGES (SUPAC) GUIDANCE FOR INDUSTRY: A REGULATORY NOTE – PHARMA
SCALE UP AND POSTAPPROVAL CHANGES (SUPAC) GUIDANCE FOR INDUSTRY: A REGULATORY NOTE – PHARMA INTRODUCTION Technology transfer of a pharmaceutical product from research to the production floor with simultaneous increase in production outputs is commonly known as scale-up. In simple terms, the process of increasing batch size is termed as …
Read More »SOP for PLASTICS AS PACKAGING MATERIAL
SOP for PLASTICS AS PACKAGING MATERIAL Plastics in packaging have proved useful for a number of reasons, including the ease with which they can be formed, their high quality, and the freedom of design to which they can be changed. Plastic containers are extremely resistant to breakage and thus offer …
Read More »SOP and Guideline for Hold-Time Studies of Tablets
SOP and Guideline for Hold-Time Studies of Tablets These guidelines focus primarily on aspects that should be considered in the design of the hold-time studies during the manufacture of non-sterile solid dosage forms. Many of the principles described here also apply to other dosage forms such as liquids, creams, and …
Read More »SOP for Hold Time Studies Protocol of Ornidazole Tablets 500 mg
SOP for Hold Time Studies Protocol of Ornidazole Tablets 500 mg Process Step – Blending/ Lubrication About 500 gms material from blended mass is kept under simulating conditions. The material is subjected to analysis for Assay of Ornidazole,Total & single impurity and LOD at 0 day, after 7th day & after 15th …
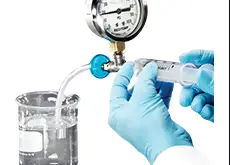
Read More »SOP FOR BUBBLE POINT TEST
SOP FOR BUBBLE POINT TEST Introduction Membrane filters have been used successfully for many years to remove yeast, bacteria and particulate from fluid streams. The ultimate integrity test for a sterilizing grade membrane filter is the bacterial challenge. Unfortunately, this is a destructive test; the filter cannot be used afterwards …
Read More »