Organization and Personnel Responsibilities of quality control unit. (a) There shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug product containers, closures, in-process materials, packaging material, labeling, and drug products, and the authority to review production records to assure …
Read More »ANVISA – The Brazilian Health Surveillance Agency
The Brazilian Health Regulatory Agency (Anvisa) is an autarchy linked to the Ministry of Health, part of the Brazilian National Health System (SUS) as the coordinator of the Brazilian Health Regulatory System (SNVS), present throughout the national territory. Anvisa’s role it to promote the protection of the population’s health by …
Read More »Vial Washing Machine: Streamlining Sterile Pharmaceutical Packaging
Vial Washing Machine: Streamlining Sterile Pharmaceutical Packaging In the pharmaceutical industry, maintaining the integrity and cleanliness of packaging materials is crucial for ensuring the safety and efficacy of drugs. Vial washing machines play a vital role in the production of sterile pharmaceuticals by efficiently cleaning and sanitizing vials before they …
Read More »New Product Design and Development Process
New Product Design and Development Process Objective To lay down a Procedure for New Product Design and Development Process. Scope This Standard Operating Procedure is applicable to pharmaceutical formulation plants. Responsibility Head, Clinical Research : Conducting Clinical Trials as per the Good Clinical Practices and review and compilation of data …
Read More »Technical Directions for Drug Product Manufacturing
Preparation, Approval, Review and Control of Technical Directions for Manufacture of Drug Products. Objective To lay down a procedure for the Preparation, Approval, Review and Control of Technical Directions for Manufacture of Drug Products. Scope This Standard Operating Procedure is applicable for preparation and implementation of Technical Directions for all …
Read More »Un-Planned Deviation -3 in Pharma
Un-Planned Deviation Details for Deviation No.______________ Department : Production Investigation starts date: Investigation completion date: INVESTIGATION TEAM S.No. Name Designation Department 1. Production Head Production 2. Production Manager Production 2. Engineer Technologist Production 4. QA Officer Quality Assurance 5. QA Head Quality Assurance Sr. No. Product Batch …
Read More »Un-Planned Deviation -II in Pharma
Un-Planned Deviation – II in Pharma Details for Deviation No. ________ Department : Production Investigation start date : Investigation completion date: INVESTIGATION TEAM S.No. Name Designation Department 1. Production Head Production 2. Production Manager Production 2. EngineerTechnologist Production 4. QAOfficer Quality Assurance 5. QA Head Quality Assurance …
Read More »Un-Planned Deviation in Pharma
Un-Planned Deviation in Pharma Investigation Report Description of Deviation: The document entry of dispensing and cleaning records is not recorded by the respective operators. Type of deviation: Un-Planned Deviation Class of Deviation: Minor Date of investigation: Department: Warehouse Date of deviation Observed: Investigations start date: Investigation completion date: INVESTIGATION TEAM …
Read More »List OF SOPs ENGINEERING
List OF SOPs ENGINEERING S. No. SOP Title 1. Preventive Maintenance 2. Breakdown Maintenance Procedure of the Equipment and Machines 3. Colour Coding of Utility Lines 4. Procedure for Building Maintenance 5. Calibration of Equipment and Instrument from External Authorized Body / Suppliers 6. Operating procedure of Vacuum Circuit Breakers …
Read More »List of SOPs of Environment, Occupational Health and Safety (EOHS)
List of SOPs of Environment, Occupational Health, and Safety (EOHS) S. No. SOP Title 1. Operating procedure for Effluent treatment plant 2. Procedure for analysis of treated effluent 3. Procedure for operation and calibration of Tds meter in ETP laboratory 4. Calibration and Operating procedure for pH meter 5. Operating …
Read More »Warehouse (Stores)
Warehouse (Stores) Warehousing is a complex and dynamic field that plays a critical role in maintaining the integrity and safety of products. Adhering to regulatory standards, and implementing best practices, Warehouses or stores can contribute to the overall efficiency of the supply chain. The Importance of Warehousing: Warehouses or stores …
Read More »HR SOPs (Human Resources)
HR SOPs (Human Resources) S. No. SOP Title 1 Medical Examination 2 Personnel clothing and Hygiene 3 First Aid Procedures 4 Accident management Procedures 5 Preparation of Job responsibilities 6 Training of Personnel s 7 Scrap Management and Disposal 8 Cleaning and Sanitation of General Area 9 Cleaning and Classification …
Read More »SOP LIST (Quality Assurance)
SOP LIST (Quality Assurance) S. No. SOP Title 1. Preparation, Approval, and Control of Standard Operating Procedures 2. Vendor Approval 3. Change Control 4. Preparation, Approval, Review and Control of Technical Directions for Manufacture of Drug Products. 5. Preparation, Approval and Control of Specifications and Standard Test Procedures …
Read More »SOP on Sampling of Raw Material
SOP on Sampling of Raw Material Objective: To lay down the procedure for the sampling of raw material. Scope: This procedure is applicable for the sampling of raw material. Responsibility: Chemist or above Accountability: Head – Quality Control Procedure: Sampling of Raw Materials: Raw material sampling shall be initiated after …
Read More »List of SOPs Quality Control
List of SOPs Quality Control Sr. No. SOP Title 1. SOP on entry & exit procedure in Quality Control Department 2. SOP on sampling of raw material. 3. SOP on intermediate and finished product analysis and approval 4. SOP on analysis of sample by contract laboratory 5. SOP on sampling …
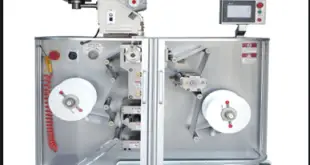
Read More »SOP for operation of Strip Packing Machine
SOP for operation of Strip Packing Machine Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of Strip Packing Machine. Scope This SOP is applicable for the operation of Strip Packing Machine used to pack the Tablets and Capsules into Strips in the formulation plant. Responsibility Production …
Read More »SOP for operation of de-dusting and polishing machine
SOP for operation of de-dusting and polishing machine Standard Operating Procedure (SOP) Objective To lay down a procedure for operation of de-dusting and polishing machine. Scope This SOP is applicable for operation of de-dusting and polishing machine used to polish the Filled Capsules in Capsule Filling Area to the formulation …
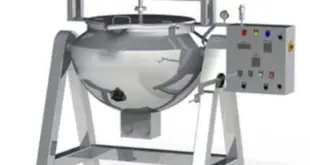
Read More »SOP on operation of Paste Kettle
SOP on operation of Paste Kettle Standard Operating Procedure (SOP) Objective To lay down the procedure for operation of Paste Kettle Scope This SOP is applicable for operation of Paste Kettle in the formulation plant. Responsibility Production Operator/ Technician – For operation of the equipment. Production Officer/ Executive – To …
Read More »SOP FOR STABILITY STUDY IN PHARMA INDUSTRY
SOP FOR STABILITY STUDY IN PHARMA INDUSTRY OBJECTIVE: To lay down the procedure for collection, storage and analysis of stability of samples. SCOPE: This SOP shall be applicable for stability study of samples in Quality Control Department in Pharma Industry. RESPONSIBILITY: Officers/Executive-Quality Control shall be responsible for follow that procedure. …
Read More »Tablets In Pharma Industry
Tablets In Pharma Industry NOTE- The provisions of this monograph do not necessarily apply to tablets intended for use other than by oral administration such as Vaginal preparations or Or mucosal preparations, and to lozenges, oral pastes and oral gums. Introduction Tablets are solid dosage forms each containing a unit …
Read More »