GUIDE TO INSPECTIONS OF HIGH PURITY WATER SYSTEMS I. SYSTEM DESIGN For parenteral products where there is a concern for pyrogens, it is expected that Water for Injection will be used, This applies to the formulation of products, as well as to the final washing of components and equipment used …
Read More »Meanings of terms
Meanings of terms Alcohol: The term “alcohol” without qualification means ethanol (95 percent). Other dilutions of ethanol are indicated by the term “ethanol” or “alcohol” followed by a statement of the percentage by volume of ethanol (CZH60) required. Desiccator: A tightly closed container of suitable size and design that maintains an …
Read More »HACCP VALIDATION REPORT
HACCP VALIDATION REPORT LIST OF CONTENTS S. No. CONTENTS PAGE No. 1.0 Objective 2.0 Scope 3.0 Reason for Validation 4.0 Responsibility 5.0 Procedure for Validation 6.0 Sampling Plan 7.0 Data Recording 8.0 Acceptance Criteria 9.0 Deviations 10.0 Conclusion and Recommendation OBJECTIVE: HACCP validation is the element of verification focused on …
Read More »Engineering Documentation in Pharmaceutical Industry
Engineering Documentation in Pharmaceutical Industry Heat Load Calculations. AHU Zoning drawing with pressure differentials. Airflow diagram. Ducting layout. Piping Layout. Hepa Box G.A. Man Movements layout Material Movements layout The layout of Pressure difference and area classification in production Area Qualification Area Mapping Commissioning Report of HVAC System Airflow & …
Read More »HPLC Chromatography
HPLC Chromatography (Questions & Answers) HPLC is a chromatographic technique that employs a liquid mobile phase to separate and analyze components of a sample. Unlike traditional liquid chromatography, HPLC utilizes high pressure to force the liquid mobile phase through a packed column, enhancing separation efficiency and speeding up analysis times. …
Read More »Dissolution (Question & Answer)
Dissolution (Question & Answer) 1. Question: What is dissolution? Answer: Dissolution is the time require to release the active drugs substances into liquid form in a dissolution medium under setup conditions (RPM, Time, and Temperature). 2. Question: What is USP chapter No. for Dissolution? Answer: USP chapter No, <711> 3. …
Read More »Interview Questions & Answers (Quality Assurance)
Interview Questions & Answers (Quality Assurance) 1.What is Quality Assurance : Quality Assurance is a deep concept covering all matters that individually or collectively influence the quality of a product. It is the complete & whole of the arrangements made with the object of ensuring that the manufactured products are of …
Read More »OOS (Out of Specification) As PER USFDA & MHRA
OOS (Out of Specification) Definition: It is defined as those results of the in-process or Finished which has been sed products testing, which falling out specified limit or acceptance Criteria which has been specified in the official Monograph or Product registered Specification. OOS was found due to the following reasons: …
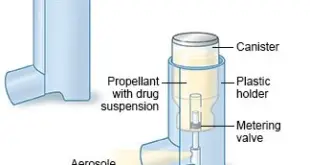
Read More »SPECIFIC REQUIREMENTS FOR MANUFACTURE OF METERED – DOSE INHALERS (MDI)
SPECIFIC REQUIREMENTS FOR MANUFACTURE OF METERED–DOSE INHALERS (MDI) General .– Manufacture of Metered-Dose-Inhalers shall be done under conditions which shall ensure minimum microbial and particulate contamination. Assurance of the quality of components and the bulk product is very important. Where medicaments are in suspended state, uniformity of suspension shall be established. …
Read More »SPECIFIC REQUIREMENTS FOR MANUFACTURE OF TOPICAL PRODUCTS
SPECIFIC REQUIREMENTS FOR MANUFACTURE OF TOPICAL PRODUCTS i.e. EXTERNAL PREPARATIONS (CREAMS, OINTMENTS, PASTES, EMULSIONS, LOTIONS, SOLUTIONS, DUSTING POWDERS, AND IDENTICAL PRODUCTS). The entrance to the area where topical products are manufactured shall be through a suitable airlock. Outside the airlock, insectocutors shall be installed. The air to this manufacturing area …
Read More »Environmental Monitoring
Environmental Monitoring – All environmental parameters listed under para 3.1 to 3.10 shall be verified and established at the time of installation and thereafter monitored at periodic intervals. The recommended frequencies of periodic monitoring shall be as follows: ( a ) Particulate monitoring in air – 6 Monthly (b) HEPA …
Read More »SPECIFIC REQUIREMENTS FOR MANUFACTURE OF ORAL LIQUIDS (SYRUPS, ELIXIRS, EMULSIONS AND SUSPENSIONS)
SPECIFIC REQUIREMENTS FOR MANUFACTURE OF ORAL LIQUIDS (SYRUPS, ELIXIRS, EMULSIONS AND SUSPENSIONS) Building And Equipment – The premises and equipment shall be designed, constructed, and maintained to suit the manufacturing of Oral Liquids. The layout and design of the manufacturing area shall strive to minimize the risk of cross-contamination and …
Read More »SPECIFIC REQUIREMENTS FOR MANUFACTURE OF ORAL SOLID DOSAGE FORMS (TABLETS AND CAPSULES)
SPECIFIC REQUIREMENTS FOR MANUFACTURE OF ORAL SOLID DOSAGE FORMS (TABLETS AND CAPSULES) The processing of dry materials and products creates problems of dust control and cross-contamination. Special attention is, therefore, needed in the design, maintenance, and use of premises and equipment in order to overcome these problems. Wherever required, enclosed …
Read More »Documentation for Manufacture of Sterile products
Documentation for Manufacture of Sterile products The manufacturing records relating to manufacture of sterile products shall indicate the following details: – (1) Serial number of the Batch Manufacturing Record. (2) Name of the product. (3) Reference to Master Formula Record. (4) Batch / Lot number. (5) Batch / Lot size. …
Read More »In-process Checks during Tertiary Packaging Operations
In-process Checks during Tertiary Packaging Operations of cartons/ shrink-wrapped units in one corrugated box / LDPE bag: Count the no. of cartons / shrink wrapped units in one corrugated box / LDPE bag and verify with the Batch Packing Record. Stencil on corrugated boxes: Check the stencil details on the …
Read More »In-process Checks during Secondary Packaging Operations
In-process Checks during Secondary Packaging Operations Performed in In-process quality checks as pre-defined frequency in BPR and to be sorted out the strips/blister with below-mentioned tablet defects before packed in carton or during Packing Broken Tablets Laminated Tablets Chipped Tablets Discoloured Tablets With Black particles Tablets With foreign particle Tablets …
Read More »In-process Checks during Primary Packaging Operations
In-process Checks during Primary Packaging Operations (Blister / Strip Packing) Record the temperature and relative humidity of the Packaging area. Tablets and Capsules: Following defect /Issue to be sorted out before strip/blister sealing Broken Tablets Laminated Tablets Chipped Tablets Discoloured Tablets With Black particles Tablets With foreign particle Tablets Distorted …
Read More »Optimal HVAC Systems for Non-Sterile Pharmaceutical Environments: Ensuring Quality and Compliance
Optimal HVAC Systems for Non-Sterile Pharmaceutical Environments: Ensuring Quality and Compliance In the pharmaceutical industry, maintaining a controlled environment is crucial to ensure product quality, safety, and regulatory compliance. HVAC (Heating, Ventilation, and Air Conditioning) systems play a vital role in non-sterile pharmaceutical facilities by providing appropriate temperature, humidity, and …
Read More »Temperature mapping of storage areas: Ensuring Reliable Environmental Control and Compliance
Temperature mapping of storage areas: Ensuring Reliable Environmental Control and Compliance In various industries, maintaining proper temperature control within critical areas is essential to ensure product quality, integrity, and regulatory compliance. Temperature mapping of storage areas is a systematic process that involves monitoring and analyzing temperature distribution within a defined …
Read More »Observations & Compliances response of GMP Inspections
Observations & Compliances response of GMP Inspections Observations 1: In the DM water plant, there were no records maintained for the change of air filters from the compressor to the degasser. Response: The procedure for the replacement of filters has been incorporated in the SOP for the replacement of cartridge …
Read More »